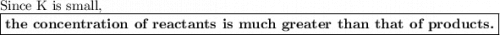Chemistry, 16.07.2019 03:10 19dansiste
The equilibrium-constant of the reaction no2(g)+no3(g)⇌n2o5(g) is k=2.1×10−20. what can be said about this reaction? a. at equilibrium the concentration of products and reactants is about the same. b. at equilibrium the concentration of products is much greater than the concentration of reactants. c. at equilibrium the concentration of reactants is much greater than that of products. d. there are no reactants left over once the reaction reaches equilibrium.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, tchase0616
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 10:00, nana54muller
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 14:00, Killion2022
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
The equilibrium-constant of the reaction no2(g)+no3(g)⇌n2o5(g) is k=2.1×10−20. what can be said abou...
Questions in other subjects:

Advanced Placement (AP), 21.04.2020 02:28



Mathematics, 21.04.2020 02:29

Computers and Technology, 21.04.2020 02:29

English, 21.04.2020 02:29



Mathematics, 21.04.2020 02:29

Mathematics, 21.04.2020 02:29

![K = \dfrac{[\text{Products}]}{[\text{Reactants}]}](/tpl/images/0094/8248/37418.png)