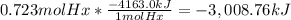Chemistry, 15.07.2019 19:30 wardlawshaliyah
The combustion of hexane is given by the following reaction. 2 c6h14 + 19 o2 12 co2 + 14 h2o the enthalpy of reaction is −4163.0 kj/mol. how much energy (in joules) will be released if 62.30 grams of hexane is burned. (molar mass of hexane = 86.20 g/mol).

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, miller5452
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1


Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1
You know the right answer?
The combustion of hexane is given by the following reaction. 2 c6h14 + 19 o2 12 co2 + 14 h2o the ent...
Questions in other subjects:


Mathematics, 28.01.2020 00:31


Mathematics, 28.01.2020 00:31




History, 28.01.2020 00:31

Geography, 28.01.2020 00:31