Chemistry, 14.07.2019 18:10 Natavia3402
Find the enthalpy of neutralization of hcl and naoh. 137 cm3 of 2.6 mol dm-3 hydrochloric acid was neutralized by 137 cm3 of 2.6 mol dm-3 naoh. the temperature rose from 298 k to 325.8 k. the specific heat capacity is the same as water, 4.18 j/k g.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, jordan5778
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 07:10, nasrul3725
Remember to use the proper number of significant figures and leading zeros in all calculations. gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 23.06.2019 01:00, only1cache
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2

Chemistry, 23.06.2019 03:00, rhianna18
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 1
You know the right answer?
Find the enthalpy of neutralization of hcl and naoh. 137 cm3 of 2.6 mol dm-3 hydrochloric acid was n...
Questions in other subjects:



Mathematics, 18.11.2019 17:31



English, 18.11.2019 17:31

Health, 18.11.2019 17:31

Mathematics, 18.11.2019 17:31


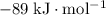 .
.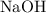 formula units will neutralize one mole of
formula units will neutralize one mole of 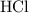 to produce one mole of water.
to produce one mole of water. 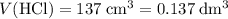 .
.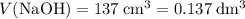 .
.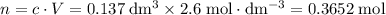 .
.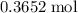 of
of 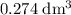 . Assume that the density of the solution is equal to that of water under room temperature.
. Assume that the density of the solution is equal to that of water under room temperature. 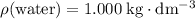 . The mass of the liquid will be
. The mass of the liquid will be 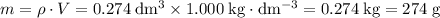 .
.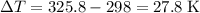 .
.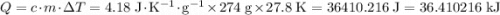 .
. .
.