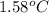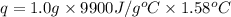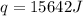Chemistry, 13.07.2019 01:30 jmanrules200
When 1.0 g of fructose, c6h12o6(s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the calorimeter increases by 1.58 °c. if the heat capacity of the calorimeter and its contents is 9.90 kj/°c, what is q for this combustion?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, yasiroarafat12
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 23.06.2019 01:00, bsheepicornozj0gc
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

Chemistry, 23.06.2019 04:20, tyrickdavis1
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1
You know the right answer?
When 1.0 g of fructose, c6h12o6(s), a sugar commonly found in fruits, is burned in oxygen in a bomb...
Questions in other subjects:

Biology, 20.10.2019 08:00

Geography, 20.10.2019 08:00





Chemistry, 20.10.2019 08:00



Mathematics, 20.10.2019 08:00

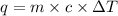
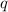 = heat of combustion = ?
= heat of combustion = ?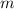 = mass of fructose = 1.0 g
= mass of fructose = 1.0 g = heat capacity of the calorimteter =
= heat capacity of the calorimteter = 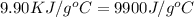
 = change in temperature =
= change in temperature =