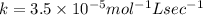Chemistry, 12.07.2019 21:30 mxddisonxo
The reaction between ethyl bromide (c2h5br) and hydroxide ion in ethyl alcohol at 330 k, c2h5br(alc) + oh-(alc) --> c2h5oh(l) + br-(alc), is first order each in ethyl bromide and hydroxide ion. when [c2h5br] is 0.0477 m and [oh-] is 0.100 m, the rate of disappearance of ethyl bromide is 1.7 x 10^-7 m/s.
what is the value of the rate constant?
k=?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, andaws21
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
The reaction between ethyl bromide (c2h5br) and hydroxide ion in ethyl alcohol at 330 k, c2h5br(alc)...
Questions in other subjects:

Mathematics, 07.10.2019 10:00



Chemistry, 07.10.2019 10:00

Mathematics, 07.10.2019 10:00

Mathematics, 07.10.2019 10:00

Mathematics, 07.10.2019 10:00


Mathematics, 07.10.2019 10:00

Biology, 07.10.2019 10:00

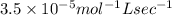
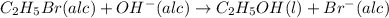
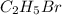 = 1
= 1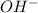 = 1
= 1![Rate=k[C_2H_5Br]^1[OH^-]^1](/tpl/images/0082/3546/7f75e.png)
![Rate=-\frac{1d[C_2H_5Br]}{dt}=k[C_2H_5Br]^1[OH^-]^1](/tpl/images/0082/3546/b56e9.png)
![\frac{d[C_2H_5]}{dt}]=1.7\times 10^{-7}](/tpl/images/0082/3546/5e05d.png)
![Rate=1.7\times 10^{-7}=k[0.0477]^1[0.100]^1](/tpl/images/0082/3546/1123d.png)