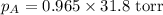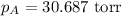Chemistry, 12.07.2019 18:10 damaricoleman42
Calculate the vapor pressure of a solution containing 24.6 g of glycerin (c3h8o3) in 134 ml of water at 30.0 ∘c. the vapor pressure of pure water at this temperature is 31.8 torr. assume that glycerin is not volatile and dissolves molecularly (i. e., it is not ionic) and use a density of 1.00 g/ml for the water.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:30, jrfranckowiak
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 19:00, elizabethajih99
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
You know the right answer?
Calculate the vapor pressure of a solution containing 24.6 g of glycerin (c3h8o3) in 134 ml of water...
Questions in other subjects:

Mathematics, 12.08.2020 04:01








Mathematics, 12.08.2020 04:01

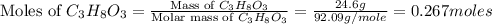
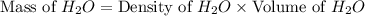
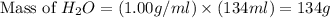
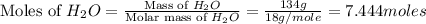
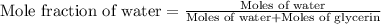
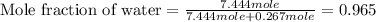
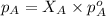
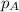 = vapor pressure of solution = ?
= vapor pressure of solution = ?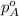 = vapor pressure of pure water= 31.8 torr
= vapor pressure of pure water= 31.8 torr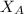 = mole fraction of water = 0.965
= mole fraction of water = 0.965