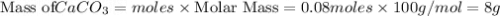Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, bakoeboo
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 04:30, ajsoccer1705
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3


Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1
You know the right answer?
60.0 ml of 3.00 m na2co3 and 40. ml of 2.00 m cacl2 were mixed together. what is the limiting reagen...
Questions in other subjects:

History, 13.10.2020 23:01





Mathematics, 13.10.2020 23:01



Mathematics, 13.10.2020 23:01

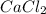 is the limiting reagent and theoretical yield of
is the limiting reagent and theoretical yield of 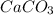 is 8 grams.
is 8 grams.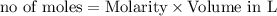
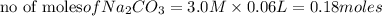
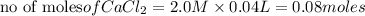
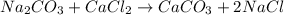
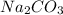 combines with 1 mole of
combines with 1 mole of 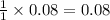 moles of
moles of