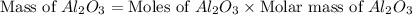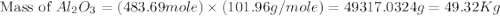Chemistry, 12.07.2019 03:20 deaerionharper
2al(s)+fe2o3(s)−→−heatal2o3(s)+2fe( l) 2al(s)+fe2o3(s)→heatal2o3(s)+2fe(l) if 26.1 kg al26.1 kg al reacts with an excess of fe2o3,fe2o3, how many kilograms of al2o3al2o3 will be produced?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:10, zwbaby3693
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 02:20, alejandraluna95
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
You know the right answer?
2al(s)+fe2o3(s)−→−heatal2o3(s)+2fe( l) 2al(s)+fe2o3(s)→heatal2o3(s)+2fe(l) if 26.1 kg al26.1 kg al r...
Questions in other subjects:


Mathematics, 28.01.2021 02:00



Mathematics, 28.01.2021 02:00

Chemistry, 28.01.2021 02:00

Mathematics, 28.01.2021 02:00

Mathematics, 28.01.2021 02:00


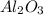 produced will be, 49.32 Kg
produced will be, 49.32 Kg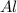 = 26.1 Kg = 26100 g
= 26.1 Kg = 26100 g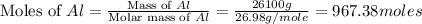
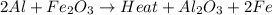
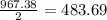 moles of
moles of