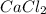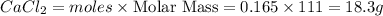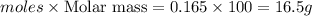When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water are produced.
caco3 + 2hcl ⟶cacl2 + h2o + co2
a) how many grams of calcium chloride will be produced when 26.0g of calcium carbonate are combined whith 12.0g of hydrochloric acid?
b) which reactant is in excess and how many grams of this reactant will remain after the reaction is complete?

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 06:00, mustafajibawi1
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1
You know the right answer?
When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water ar...
Questions in other subjects:


Mathematics, 08.08.2019 22:20

Mathematics, 08.08.2019 22:20

English, 08.08.2019 22:20






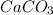 is the excess reagent and 16.5g of
is the excess reagent and 16.5g of 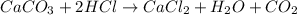
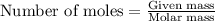
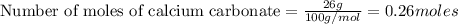
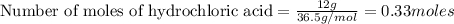
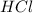 react with 1 mole of
react with 1 mole of 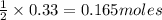 of
of