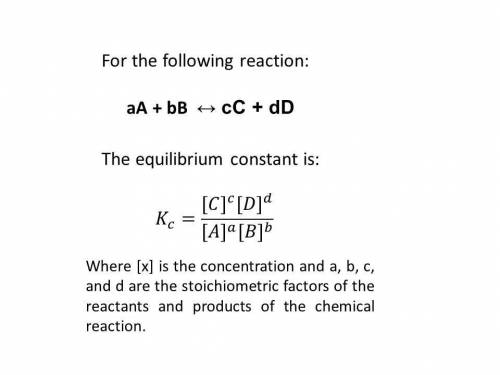
Consider the following reaction and its equilibrium constant: 4 cuo(s) + ch4(g) ⇌ co2(g) + 4 cu(s) + 2 h2o(g) kc = 1.10.
a reaction mixture contains 0.22 m ch4, 0.70 m co2 and 1.5 m h2o. which of the following statements is true concerning this system? a. the reaction will shift in the direction of products. b. the equilibrium constant will increase. c. the reaction will shift in the direction of reactants. d. the system is at equilibrium.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, Slycooper5959
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 06:00, joelpimentel
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 18:10, NEONREDBLADE
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 22.06.2019 21:30, steven0448
An atomic nucleus is composed ofa)protons. b)protons and neutrons. c)protons and electrons. d)protons, neutrons, and electrons.
Answers: 1
You know the right answer?
Consider the following reaction and its equilibrium constant: 4 cuo(s) + ch4(g) ⇌ co2(g) + 4 cu(s)...
Questions in other subjects:


Mathematics, 20.05.2021 16:50


Mathematics, 20.05.2021 16:50

Mathematics, 20.05.2021 16:50

Mathematics, 20.05.2021 16:50


Business, 20.05.2021 16:50

Mathematics, 20.05.2021 16:50

![Q = \frac{[CO_{2}][H_{2}O]^{2}}{[CH_{4}]}](/tpl/images/0072/0904/ac4b6.png) (2)
(2)![Q = \frac{[CO_{2}][H_{2}O]^{2}}{[CH_{4}]} = \frac{0.70*(1.5)^{2}}{0.22} = 7.16](/tpl/images/0072/0904/5a31b.png)