
Chemistry, 09.07.2019 01:30 glydelxc2780
Calculate the solubility of nitrogen in water at an atmospheric pressure of 0.480 atm (a typical value at high altitude). atmospheric gas mole fraction kh mol/(l*atm) n2 7.81 x 10-1 6.70 x 10-4 o2 2.10 x 10-1 1.30 x 10-3 ar 9.34 x 10-3 1.40 x 10-3 co2 3.33 x 10-4 3.50 x 10-2 ch4 2.00 x 10-6 1.40 x 10-3 h2 5.00 x 10-7 7.80 x 10-4

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1



Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
You know the right answer?
Calculate the solubility of nitrogen in water at an atmospheric pressure of 0.480 atm (a typical val...
Questions in other subjects:





History, 22.06.2019 16:00

English, 22.06.2019 16:00




English, 22.06.2019 16:00


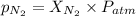
 = partial pressure of nitrogen = ?
= partial pressure of nitrogen = ? = mole fraction of nitrogen =
= mole fraction of nitrogen = 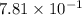
 = atmospheric pressure = 0.480 atm
= atmospheric pressure = 0.480 atm


 = solubility of nitrogen in water = ?
= solubility of nitrogen in water = ? = Henry's constant =
= Henry's constant = 




