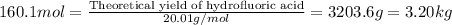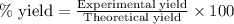Chemistry, 09.07.2019 00:50 robertschulte116
Hydrogen fluoride is used in the manufacture of freons (which destroy ozone in the stratosphere) and in the production of aluminum metal. it is prepared by the reaction caf2 + h2so4 → caso4 + 2hf in one process, 6.25 kg of caf2 is treated with an excess of h2so4 and yields 2.35 kg of hf. calculate the percent yield of hf. % yield

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, amylumey2005
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 19:00, Farhan54019
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1


You know the right answer?
Hydrogen fluoride is used in the manufacture of freons (which destroy ozone in the stratosphere) and...
Questions in other subjects:

Spanish, 03.10.2019 08:20

English, 03.10.2019 08:20

Mathematics, 03.10.2019 08:20

English, 03.10.2019 08:20


Mathematics, 03.10.2019 08:20

English, 03.10.2019 08:20


Arts, 03.10.2019 08:20

English, 03.10.2019 08:20

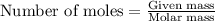 ....(1)
....(1) 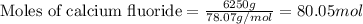
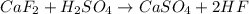
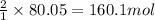 of hydrofluoric acid
of hydrofluoric acid