
Chemistry, 08.07.2019 23:40 sairaanwar67
For the reaction h2(g) + co2(g) ⇌ h2o(g) + co(g) at 700ºc, kc = 0.534. calculate the number of moles of h2 that are present at equilibrium if a mixture of 0.300 mole of co and 0.300 mole of h2o is heated to 700ºc in a 10.0-l container.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, petriajack8375
1) in saturated limewater, [h+ ]=3.98x10-13 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 2) in butter, [h+ ]=6.0x10-7 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 3) in peaches, [oh]=3.16x10-11 m a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 4) during the course of the day, human saliva varies between being acidic and basic. if [oh]=3.16x10-8 m, a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? /
Answers: 3

Chemistry, 22.06.2019 02:10, apowers6361
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3


Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1
You know the right answer?
For the reaction h2(g) + co2(g) ⇌ h2o(g) + co(g) at 700ºc, kc = 0.534. calculate the number of moles...
Questions in other subjects:

History, 05.11.2020 18:10



Mathematics, 05.11.2020 18:10




History, 05.11.2020 18:10


Mathematics, 05.11.2020 18:10

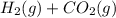 ⇄
⇄ 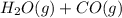 at 700ºC.
at 700ºC.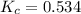
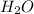 is heated to 700ºC.
is heated to 700ºC.![[H_2O]=\frac{300 mol}{10.0L}=30 M](/tpl/images/0067/3669/12641.png)
![H_2O=[H_2O]=\frac{300 mol}{10.0L}=30 M](/tpl/images/0067/3669/82ae5.png)
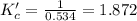
![K_c'=\frac{[H_2][CO_2]}{[CO][H_2O]}](/tpl/images/0067/3669/df550.png)
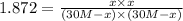
![[H_2]](/tpl/images/0067/3669/08a38.png)
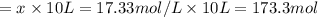
![[H_2]_{eq}=0.0173M](/tpl/images/0067/3669/87baf.png)
![Kc=\frac{[H_2O][CO]}{[H_2][CO_2]}](/tpl/images/0067/3669/4d854.png)
![[CO_2]_0=[H_2]_0=\frac{0.300mol}{10.0L} =0.030M](/tpl/images/0067/3669/89e63.png)
 due to the equilibrium, the law of mass action takes the following form:
due to the equilibrium, the law of mass action takes the following form: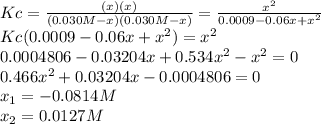
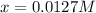
![[H_2]_{eq}=0.030M-0.0127M=0.0173M](/tpl/images/0067/3669/92f4c.png)


