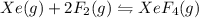Chemistry, 08.07.2019 18:10 alemorachis49
1) consider the following reaction at equilibrium. what effect will reducing the pressure of the reaction mixture have on the system? xe(g) + 2 f2(g) ? xef4(g) a)the equilibrium constant will decrease. b)no effect will be observed. c)the reaction will shift to the right in the direction of products. d) the equilibrium constant will increase e) the reaction will shift to the left in the direction of reactants.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, girlwholikesanime
Where are each of the three particles located within the atom?
Answers: 1

Chemistry, 22.06.2019 00:00, tahjaybenloss16
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 13:00, devontemiles8868
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1
You know the right answer?
1) consider the following reaction at equilibrium. what effect will reducing the pressure of the rea...
Questions in other subjects:

Mathematics, 09.11.2020 04:50



Social Studies, 09.11.2020 04:50



Mathematics, 09.11.2020 04:50



Mathematics, 09.11.2020 04:50