
Chemistry, 05.02.2020 13:46 bettinger6525
In the haber reaction, patented by german chemist fritz haber in 1908, dinitrogen gas combines with dihydrogen gas to produce gaseous ammonia. this reaction is now the first step taken to make most of the world's fertilizer.
suppose a chemical engineer studying a new catalyst for the haber reaction finds that 505 liters per second of dinitrogen are consumed when the reaction is run at 172 oc and 0.88 atm. calculate the rate at which ammonia is being produced. give your answer in kilograms per second. be sure your answer has the correct number of significant digits.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, lrasanaoaksandfurana
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1



Chemistry, 23.06.2019 04:40, dd123984
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1
You know the right answer?
In the haber reaction, patented by german chemist fritz haber in 1908, dinitrogen gas combines with...
Questions in other subjects:

Mathematics, 30.03.2020 20:15

Mathematics, 30.03.2020 20:15

Health, 30.03.2020 20:15


Mathematics, 30.03.2020 20:15

Mathematics, 30.03.2020 20:15

Mathematics, 30.03.2020 20:15


Medicine, 30.03.2020 20:16

 Haber reaction
Haber reaction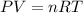
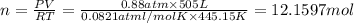
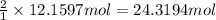 of ammonia
of ammonia

