
Chemistry, 05.02.2020 11:50 abdullaketbi71
The depletion of ozone (o3) in the stratosphere has been a matter of great concern among scientists in recent years. it is believed that ozone can react with nitric oxide (no) that is discharged from the high-altitude jet plane, the sst. the reaction is
o3 + no > o2 + no2
if 0.827 g of o3 reacts with 0.635 g of no, how many grams of no2 will be produced? g no2 which compound is the limiting reagent? ozone (o3) nitric oxide (no) calculate the number of moles of the excess reagent remaining at the end of the reaction.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:30, pookie879
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3


Chemistry, 22.06.2019 23:00, hailey5campbelp7d1c0
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 23.06.2019 01:30, sleimanabir
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2
You know the right answer?
The depletion of ozone (o3) in the stratosphere has been a matter of great concern among scientists...
Questions in other subjects:




Mathematics, 23.04.2020 02:53

English, 23.04.2020 02:53

Mathematics, 23.04.2020 02:53

Health, 23.04.2020 02:53


English, 23.04.2020 02:53

Mathematics, 23.04.2020 02:53

 ....(1)
....(1)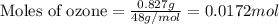
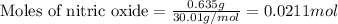
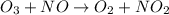
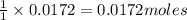 of nitric oxide
of nitric oxide


