
For each of the following acid-base reactions, calculate how many grams of each acid are necessary to completely react with and neutralize 2.7 g of the base. part a : hcl(aq)+naoh(aq)→h2o(l)+nacl(aq)par t b : 2hno3(aq)+ca(oh)2(aq)→2h2o(l)+ca(no 3)2(aq)part c : h2so4(aq)+2koh(aq)→2h2o(l)+k2so4(aq )

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:10, ellemarshall13
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
For each of the following acid-base reactions, calculate how many grams of each acid are necessary t...
Questions in other subjects:

Biology, 10.11.2021 01:20


Social Studies, 10.11.2021 01:20



Chemistry, 10.11.2021 01:20

Chemistry, 10.11.2021 01:20

Physics, 10.11.2021 01:20

Business, 10.11.2021 01:20

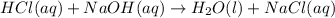
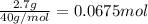
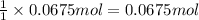 of HCl.
of HCl.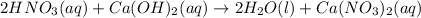
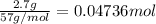
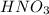 neutralizes with 1 mol of
neutralizes with 1 mol of 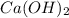 .
.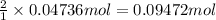 of
of 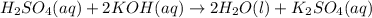
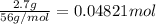
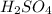 neutralizes with 2 mol of
neutralizes with 2 mol of 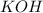 .
.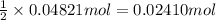 of
of 


