
Chemistry, 06.07.2019 03:20 yazmineespinozarive
Determine the amount of heat (in kj) given off when 1.26 × 104 g of ammonia are produced according to the equation n2(g) + 3h2(g) ⟶ 2nh3(g) δh°rxn = −92.6 kj/mol assume that the reaction takes place under standardstate conditions at 25°c.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1


Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
Determine the amount of heat (in kj) given off when 1.26 × 104 g of ammonia are produced according t...
Questions in other subjects:





Mathematics, 19.01.2021 06:40


English, 19.01.2021 06:40


Mathematics, 19.01.2021 06:40

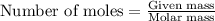
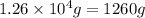
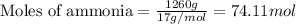
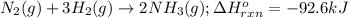
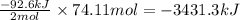 of energy.
of energy.

