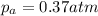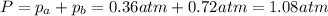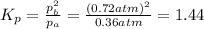Chemistry, 06.07.2019 02:20 wannaoneisforever
Consider the hypothetical reaction a((g). a flask is charged with 0.73atm of pure a, after which it is allowed to reach equilibrium at 0 ? c. at equilibrium the partial pressure of a is 0.37atm .
a: what is the total pressure in the flask at equilibrium?
b: what is the value of kp?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 09:00, 2024cynthiatercero
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
Consider the hypothetical reaction a((g). a flask is charged with 0.73atm of pure a, after which it...
Questions in other subjects:

Mathematics, 01.07.2020 15:01





Mathematics, 01.07.2020 15:01



Mathematics, 01.07.2020 15:01

 .
.