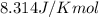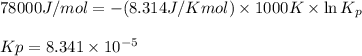Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, carter1809
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2


Chemistry, 22.06.2019 16:00, winnie45
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
The free energy of formation of nitric oxide, no, at 1000 k (roughly the temperature in an automobil...
Questions in other subjects:


English, 28.03.2020 00:38

History, 28.03.2020 00:38

Mathematics, 28.03.2020 00:38

Mathematics, 28.03.2020 00:38





Social Studies, 28.03.2020 00:38

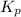 for the chemical equation is
for the chemical equation is 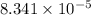
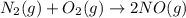
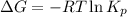
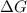 = Gibbs free energy = 78 kJ/mol = 78000 J/mol (Conversion factor: 1kJ = 1000J)
= Gibbs free energy = 78 kJ/mol = 78000 J/mol (Conversion factor: 1kJ = 1000J)