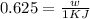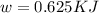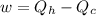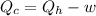Chemistry, 05.07.2019 22:30 bapefer498
Acertain heat engine operates between 800 k and 300 k. (a) what is the maximum efficiency of the engine? (b) calculate the maximum work that can be done by for each 1.0 k) of hea a reversible process for each 1.0 kj supplied by the hot source? t supplied by the hot source. (c) how much heat is discharged into the cold sink in

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, mommatann
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 10:10, ragegamer334p3xlso
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 18:30, robjaykay
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2

Chemistry, 22.06.2019 20:50, iluminatioffial9699
One nanometer is equal to how many meters?
Answers: 2
You know the right answer?
Acertain heat engine operates between 800 k and 300 k. (a) what is the maximum efficiency of the eng...
Questions in other subjects:


Mathematics, 03.01.2021 07:00



Mathematics, 03.01.2021 07:00





Mathematics, 03.01.2021 07:00

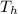 = 800 K
= 800 K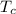 = 300 K
= 300 K

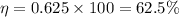
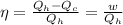
 = heat supplied by hot source = 1 KJ
= heat supplied by hot source = 1 KJ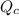 = heat supplied by hot source
= heat supplied by hot source