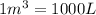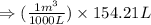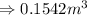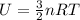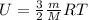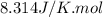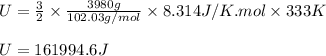Chemistry, 05.07.2019 22:20 jackiecroce1
Arigid vessel contains 3.98 kg of refrigerant-134a at 700 kpa and 60°c. determine the volume of the vessel and the total internal energy. m3 (round to four decimal places) kj (round to one decimal place)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, rightstrong9827
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1


Chemistry, 22.06.2019 22:30, angelagonzalesownus1
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
Arigid vessel contains 3.98 kg of refrigerant-134a at 700 kpa and 60°c. determine the volume of the...
Questions in other subjects:


Mathematics, 23.12.2020 23:40



Geography, 23.12.2020 23:40


Chemistry, 23.12.2020 23:40

Biology, 23.12.2020 23:40

Mathematics, 23.12.2020 23:40

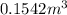 and total internal energy is 162.0 kJ.
and total internal energy is 162.0 kJ. 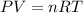
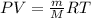
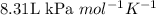
![60^oC=[60+273]K=333K](/tpl/images/0055/6153/72ddf.png)
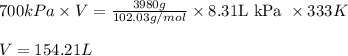
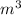 , we use the conversion factor:
, we use the conversion factor: