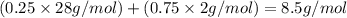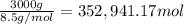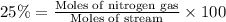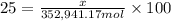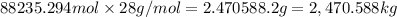Chemistry, 05.07.2019 22:10 tarhondaeiland4122
The feed to an ammonia synthesis reactor contains 25 mole% nitrogen and the balance hydrogen. the fow rate of the stream is 3000 kg/h. calculate the rate of flow of nitrogen into the reactor in kg/h. (suggestion: first calculate the average molecular weight of the mixture.)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:20, anggar20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 12:40, valenzueladomipay09u
How does concentration affect reaction rate
Answers: 2

Chemistry, 22.06.2019 14:00, JJlover1892
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2
You know the right answer?
The feed to an ammonia synthesis reactor contains 25 mole% nitrogen and the balance hydrogen. the fo...
Questions in other subjects:


Mathematics, 27.02.2020 19:13