
The equilibrium constant, kc, for the following reaction is 9.52×10-2 at 350 k. ch4 (g) + ccl4 (g) 2 ch2cl2 (g) calculate the equilibrium concentrations of reactants and product when 0.377 moles of ch4 and 0.377 moles of ccl4 are introduced into a 1.00 l vessel at 350 k.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:00, coolkid2041
Calculate the number of moles of ethane in 100 grams
Answers: 3

Chemistry, 22.06.2019 07:30, deidaraXneji
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3
You know the right answer?
The equilibrium constant, kc, for the following reaction is 9.52×10-2 at 350 k. ch4 (g) + ccl4 (g) 2...
Questions in other subjects:

Mathematics, 14.12.2020 22:20





Mathematics, 14.12.2020 22:20


Arts, 14.12.2020 22:20


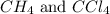 is 0.377 M and equilibrium concentration of
is 0.377 M and equilibrium concentration of 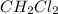 is 0.116 M
is 0.116 M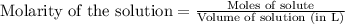
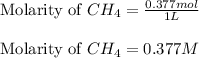

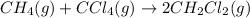
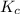 for the given equation follows:
for the given equation follows:![K_c=\frac{[CH_2Cl_2]^2}{[CH_4][CCl_4]}](/tpl/images/0055/3628/bf52a.png)
![K_c=9.52\times 10^{-2}\\[CH_4]=0.377M\\[CCl_4]=0.377M](/tpl/images/0055/3628/8ee11.png)
![9.52\times 10^{-2}=\frac{[CH_2Cl_2]^2}{(0.377)\times (0.377)}](/tpl/images/0055/3628/5aafe.png)
![[CH_2Cl_2]=0.116M](/tpl/images/0055/3628/7ed7d.png)


