The equilibrium constant, kc, for the reaction
n2o4(g)? 2no2(g)
is 4.9×10? 3.
if t...

Chemistry, 05.07.2019 20:20 ronaldhernandez598
The equilibrium constant, kc, for the reaction
n2o4(g)? 2no2(g)
is 4.9×10? 3.
if the equilibrium mixture contains [no2] = 0.050 m , what is the molar concentration of n2o4?
express the concentration to two significant figures and include the appropriate units.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3


Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 10.03.2020 17:35









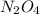 is,
is, 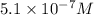

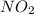 = 0.050 M
= 0.050 M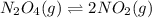
![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0055/3008/271f5.png)
![4.9\times 10^3=\frac{(0.050)^2}{[N_2O_4]}](/tpl/images/0055/3008/a5c3c.png)
![[N_2O_4]=5.1\times 10^{-7}M](/tpl/images/0055/3008/c9f7d.png)



