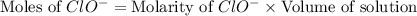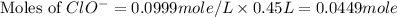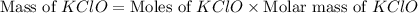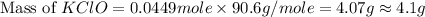Chemistry, 05.07.2019 20:20 TheJanko4526
What mass of potassium hypochlorite (fw-90.6 g/mol) must be added to 4.50 x 10 ml of water to give a solution with ph 10.20? [ka(hcio) 4.0 x 10-8] 0.032g ? 2.4 g 04.1 g 9.1 g 20. g

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, anamaliiow
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1


Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
What mass of potassium hypochlorite (fw-90.6 g/mol) must be added to 4.50 x 10 ml of water to give a...
Questions in other subjects:





Mathematics, 20.06.2020 20:57


English, 20.06.2020 20:57



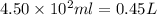
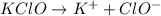
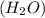 to give,
to give,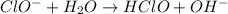
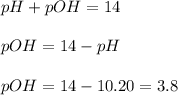
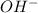 concentration.
concentration.![pOH=-\log [OH^-]](/tpl/images/0055/2981/1fac1.png)
![3.8=-\log [OH^-]](/tpl/images/0055/2981/a714c.png)
![[OH^-]=1.58\times 10^{-4}M](/tpl/images/0055/2981/110da.png)
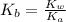
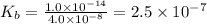
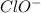 .
.![K_b=\frac{[OH^-][HClO]}{[ClO^-]}](/tpl/images/0055/2981/9b5c8.png)
![[OH^-]=[HClO]=1.58\times 10^{-4}M](/tpl/images/0055/2981/ec220.png)
![2.5\times 10^{-7}=\frac{(1.58\times 10^{-4})^2}{[ClO^-]}](/tpl/images/0055/2981/2ef50.png)
![[ClO^-]=0.0999M](/tpl/images/0055/2981/776df.png)