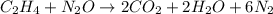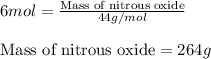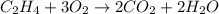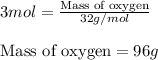Write the balanced chemical equation for the complete, stoichiometric combustion of ethylene in (a) nitrous oxide and (b) air. compare the required number of moles and the oxidizer mass using each of the two oxidizers for the complete, stoichiometric combustion of ethylene.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, eamccoy1
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2


Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

You know the right answer?
Write the balanced chemical equation for the complete, stoichiometric combustion of ethylene in (a)...
Questions in other subjects:



Mathematics, 11.11.2019 01:31

Mathematics, 11.11.2019 01:31


Mathematics, 11.11.2019 01:31




History, 11.11.2019 01:31

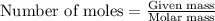 ....(1)
....(1)