
Chemistry, 05.07.2019 19:10 daniiltemkin20
The equilibrium constant for the reaction agbr(s) picture ag+(aq) + br− (aq) is the solubility product constant, ksp = 7.7 × 10−13 at 25°c. calculate δg for the reaction when [ag+] = 1.0 × 10−2 m and [br-] = 1.0 × 10−3 m. is the reaction spontaneous or nonspontaneous at these concentrations?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, DarcieMATHlin2589
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1


Chemistry, 22.06.2019 02:50, giiffnlojd
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3
You know the right answer?
The equilibrium constant for the reaction agbr(s) picture ag+(aq) + br− (aq) is the solubility produ...
Questions in other subjects:

English, 13.04.2020 20:21

Mathematics, 13.04.2020 20:21

Mathematics, 13.04.2020 20:21

Social Studies, 13.04.2020 20:21

Social Studies, 13.04.2020 20:21



Health, 13.04.2020 20:21

History, 13.04.2020 20:21

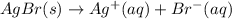
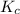 :
:![K_c=\frac{[Ag^+][Br^-]}{[AgCl]}=\frac{[Ag^+][Br^-]}{1}=[Ag^+][Br^-]](/tpl/images/0055/1226/d9269.png)
![K_{sp}=[Ag^+][Br^-]=K_c=7.7\times 10^{-13}](/tpl/images/0055/1226/964b9.png)
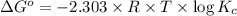
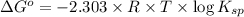
![\Delta G^o=-2.303\times 8.314 J/K mol\times 298 K\times \log[7.7\times 10^{-13}]](/tpl/images/0055/1226/37a54.png)
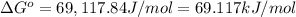
![[Ag^+] = 1.0\times 10^{-2} M](/tpl/images/0055/1226/88952.png) and
and ![[Br^-] = 1.0\times 10^{-3} M](/tpl/images/0055/1226/50b47.png)
![Q=[Ag^+][Br^-]=1.0\times 10^{-2} M\times 1.0\times 10^{-3} M=1.0\times 10^{-5}](/tpl/images/0055/1226/4f48a.png)
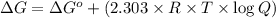
![\Delta G=69.117 kJ/mol+(2.303\times 8.314 Joule/mol K\times 298 K\times \log[1.0\times 10^{-5}])](/tpl/images/0055/1226/947e2.png)
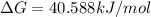
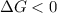 .For reaction to non spontaneous reaction:
.For reaction to non spontaneous reaction: 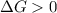 .
.

