
Chemistry, 05.07.2019 18:30 bikkiecuanas13
Be sure to answer all parts. one gallon of gasoline in an automobiles engine produces on average 9.50 kg of carbon dioxide, which is a greenhouse gas; that is, it promotes the warming of earth's atmosphere. calculate the annual production of carbon dioxide in kilograms if there are exactly 40.0 million cars in the united states and each car covers a distance of 5790 mi at a consumption rate of 24.1 miles per gallon. enter your answer in scientific notation. × 10 kg

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, andrejr0330jr
What is the molar mass of potassium nitrate, kno3
Answers: 1


Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 19:00, cindyroxana229
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
Be sure to answer all parts. one gallon of gasoline in an automobiles engine produces on average 9.5...
Questions in other subjects:


English, 28.04.2022 16:50


Business, 28.04.2022 17:10

Chemistry, 28.04.2022 17:20

Mathematics, 28.04.2022 17:20


Mathematics, 28.04.2022 17:30

Mathematics, 28.04.2022 17:30

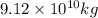 .
.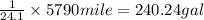
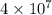
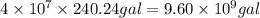
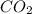
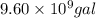 of gasoline will produce:
of gasoline will produce: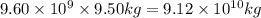 of
of 

