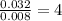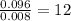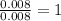Chemistry, 05.07.2019 18:20 hallmansean04
Asample containing only carbon, hydrogen, and silicon is subjected to elemental analysis. after complete combustion, a 0.7020 g sample of the compound yields 1.4 g of co2, 0.86 g of h2o, and 0.478 g of sio2. what is the empirical formula of the compound?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:50, ayoismeisjjjjuan
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x. xx ml
Answers: 1

Chemistry, 21.06.2019 23:00, dice50
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 14:30, srutkowske1489
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 20:00, aksambo4707
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
Asample containing only carbon, hydrogen, and silicon is subjected to elemental analysis. after comp...
Questions in other subjects:

Biology, 04.04.2021 01:20



Mathematics, 04.04.2021 01:20

Mathematics, 04.04.2021 01:20





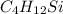 .
.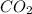 = 1.4 g
= 1.4 g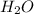 = 0.86 g
= 0.86 g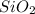 = 0.478 g
= 0.478 g 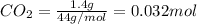
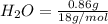 =0.048mol
=0.048mol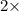 Moles of
Moles of 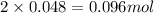
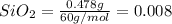 mol
mol