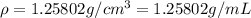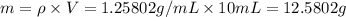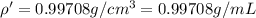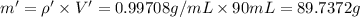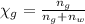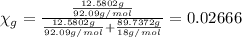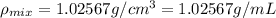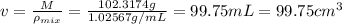You mix 10 ml glycerol and 90 ml water to obtain a 10% glycerol solution. the density of the mixture is ρmix = 1.02567 g/cm. what are the mole fraction of glycerol and the volume of the mixture? what is the reason for the volume change? mm(glycerol) = 92.09 g/mol, mm(h2o) = 18 g/mol, ρ(glycerol) = 1.25802 g/cm3, ρ(h2o) = 0.99708 g/cm3.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 23:00, genyjoannerubiera
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3
You know the right answer?
You mix 10 ml glycerol and 90 ml water to obtain a 10% glycerol solution. the density of the mixture...
Questions in other subjects:



English, 16.06.2020 20:57

Mathematics, 16.06.2020 20:57


History, 16.06.2020 20:57

Mathematics, 16.06.2020 20:57


English, 16.06.2020 20:57

Mathematics, 16.06.2020 20:57