
The chemical formula for ferric sulfate is fe(so4)3. determine the following:
a) the number of sulfur atoms in 1.75 mole of fe(so4)3
b) the mass in grams of 2.65 mol of fe(so4)3
c) the number of moles of fe(so4)3 in 3.45 grams of fe(so4)3.
d)the mass in grams of 3 formula unit of fe(so4)3

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1


Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
The chemical formula for ferric sulfate is fe(so4)3. determine the following:
a) the nu...
a) the nu...
Questions in other subjects:


English, 05.03.2021 01:00

History, 05.03.2021 01:00

Mathematics, 05.03.2021 01:00



Mathematics, 05.03.2021 01:00

Mathematics, 05.03.2021 01:00


 .
. is, 1059.682 grams.
is, 1059.682 grams.

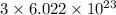 number of sulfur atoms.
number of sulfur atoms. number of sulfur atoms.
number of sulfur atoms.
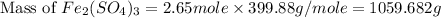


 formula unit.
formula unit.





