
Chalk is impure calcium carbonate. the amount of calcium carbonate present can be determined by hydrochloric acid to a sample of chalk and measuring the volume of carbon dioxide produced caco3(aq) + 2hcl -> cacl2(aq) + co2(g) + h2o(g) excess hydrochloric acid was added to 0.5g chalk and 100cm3 of carbon dioxide gas was given produced at r. t.p calculate the percentage purity of calcium carbonate in sample of chalk

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, tdowling331
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 04:00, armahoney8566
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 15:00, Zagorodniypolina5
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
Chalk is impure calcium carbonate. the amount of calcium carbonate present can be determined by hydr...
Questions in other subjects:


Health, 16.11.2020 23:40

Physics, 16.11.2020 23:40




Advanced Placement (AP), 16.11.2020 23:40

Health, 16.11.2020 23:40


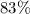 .
.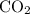 gas are released?
gas are released?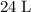 under room temperature and pressure (r.t.p,
under room temperature and pressure (r.t.p, 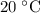 ,
, 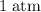 .) That's the same as
.) That's the same as 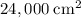 .
.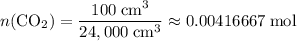 .
.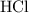 is in excess. How many moles of
is in excess. How many moles of 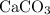 formula units will produce that
formula units will produce that 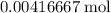 of
of 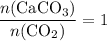 .
.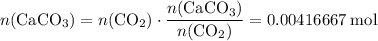 .
.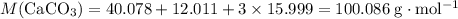 .
.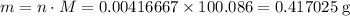 .
.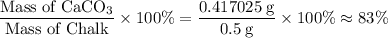 .
.

