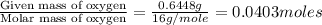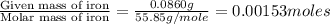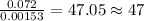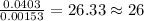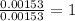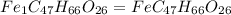Chemistry, 03.07.2019 21:20 delanieloya
When 1.6968 g of an organic iron compound containing fe, c, h, and o was burned in o2, 3.1737 g of co2 and 0.90829 g of h2o were produced. in a separate experiment to determine the mass percent of iron, 0.5446 g of the compound yielded 0.1230 g of fe2o3. what is the empirical formula of the compound?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, carsonjohnsonn
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 09:00, tashaunalewis4786
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
When 1.6968 g of an organic iron compound containing fe, c, h, and o was burned in o2, 3.1737 g of c...
Questions in other subjects:


Mathematics, 01.11.2019 23:31


Mathematics, 01.11.2019 23:31

Biology, 01.11.2019 23:31

Mathematics, 01.11.2019 23:31

Mathematics, 01.11.2019 23:31


English, 01.11.2019 23:31

Health, 01.11.2019 23:31


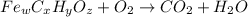
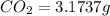

 of carbon will be contained.
of carbon will be contained. of hydrogen will be contained.
of hydrogen will be contained. =
= 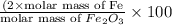

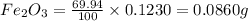 of iron.
of iron.