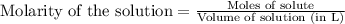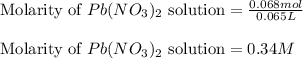Chemistry, 03.07.2019 20:30 ronaldhernandez598
Asolution of nacl(aq) is added slowly to a solution of lead nitrate, pb(no3)2(aq) , until no further precipitation occurs. the precipitate is collected by filtration, dried, and weighed. a total of 18.86 g pbcl2(s) is obtained from 200.0 ml of the original solution. calculate the molarity of the pb(no3)2(aq) solution.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 14:00, daniel1480
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 23.06.2019 00:30, coralaguilar1702
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
You know the right answer?
Asolution of nacl(aq) is added slowly to a solution of lead nitrate, pb(no3)2(aq) , until no further...
Questions in other subjects:

Biology, 04.05.2020 23:10


Health, 04.05.2020 23:10

Computers and Technology, 04.05.2020 23:10

Mathematics, 04.05.2020 23:10



English, 04.05.2020 23:10

Mathematics, 04.05.2020 23:10

History, 04.05.2020 23:10

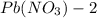 solution is 0.34 M.
solution is 0.34 M.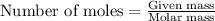
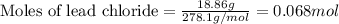
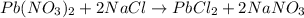
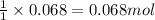 of lead nitrate
of lead nitrate