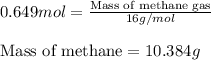Consider the following reaction: 2ch3oh(g) 2ch4(g) + o2(g) δh = +252.8 kj a) calculate the amount of heat transferred when 24.0 g of ch3oh(g) is decomposed by this reaction at constant pressure. b) for a given sample of ch3oh, the enthalpy change during the reaction is 82.1 kj. how many grams of methane gas are produced?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 18:00, ameliaxbowen7
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 23:00, lilsnsbsbs
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
Consider the following reaction: 2ch3oh(g) 2ch4(g) + o2(g) δh = +252.8 kj a) calculate the amount...
Questions in other subjects:






Mathematics, 23.11.2019 14:31




Mathematics, 23.11.2019 14:31

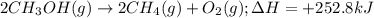
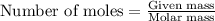 ......(1)
......(1)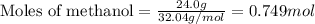
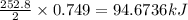
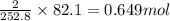 of methane gas is produced.
of methane gas is produced.