
Chemistry, 02.07.2019 19:20 fatherbamboo
The reaction between potassium chlorate (kcio,) and red phosphorus (p.) takes place when one strikes a match. the products of the reaction are tetraphosphorus decoxide and potassium chloride. if 56.0 grams of kcio, are reacted with an excess amount of red phosphorus, how many grams of p0o and kci can be produced? how much red phosphorus is consumed in the reaction? (15 pts) write the balanced reaction first!

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, zitterkoph
Which of the following mining methods disrupts the sea floor?
Answers: 1

Chemistry, 22.06.2019 04:10, tishfaco5000
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 04:30, coryoddoc3685
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 08:00, celestemaria0727
What are the similarities of physical and chemical change ?
Answers: 1
You know the right answer?
The reaction between potassium chlorate (kcio,) and red phosphorus (p.) takes place when one strikes...
Questions in other subjects:

Mathematics, 03.06.2021 18:30




Mathematics, 03.06.2021 18:30

Biology, 03.06.2021 18:30



Mathematics, 03.06.2021 18:30

Biology, 03.06.2021 18:30

 ....(1)
....(1)

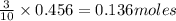 of tetraphosphorus decoxide
of tetraphosphorus decoxide
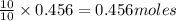 of potassium chloride
of potassium chloride
 of red phosphorus
of red phosphorus


