
Chemistry, 02.07.2019 19:10 silviamgarcia
At 35°c, kc = 1.6 multiplied by10-5 for the following reaction
2 nocl(g) reverse reaction arrow 2 no(g)+ cl2(g)
calculate the concentrations of all species at equilibrium if
2.0 mol no and 1.0 mol of cl2 are placed in a 1.0 l flask

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:40, justicejesusfreak
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1


Chemistry, 22.06.2019 20:10, sarahalexa19
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
At 35°c, kc = 1.6 multiplied by10-5 for the following reaction
2 nocl(g) reverse reaction arro...
2 nocl(g) reverse reaction arro...
Questions in other subjects:


Mathematics, 08.12.2021 02:40

Mathematics, 08.12.2021 02:40

Spanish, 08.12.2021 02:40



Mathematics, 08.12.2021 02:40


Biology, 08.12.2021 02:40

Mathematics, 08.12.2021 02:40

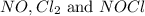 are, 0.05 M, 0.043 M and 0.975 M respectively.
are, 0.05 M, 0.043 M and 0.975 M respectively.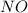 = 2 mole
= 2 mole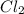 = 1 mole
= 1 mole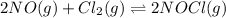
![K_c=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0043/6110/56950.png)
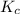 for reverse reaction =
for reverse reaction = 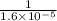
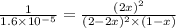
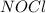 = x M = 0.975 M
= x M = 0.975 M

