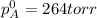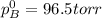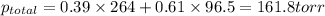Chemistry, 02.07.2019 04:10 NikkiZoeller
Liquid a has a vapor pressure of 264 torr at 20∘c, and liquid b has a vapor pressure of 96.5 torr at the same temperature. if 5.50 moles of liquid a and 8.50 moles of liquid b are combined to form an ideal solution, what is the total vapor pressure (in torr) above the solution at 20.0∘c?

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 15:40, alleshia2007
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
Liquid a has a vapor pressure of 264 torr at 20∘c, and liquid b has a vapor pressure of 96.5 torr at...
Questions in other subjects:

Mathematics, 22.04.2021 21:20



Mathematics, 22.04.2021 21:20


English, 22.04.2021 21:20

Mathematics, 22.04.2021 21:20



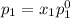 and
and 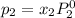
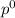 = pressure in the pure state
= pressure in the pure state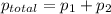
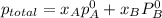
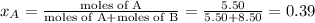 ,
, 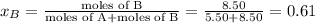 ,
,