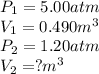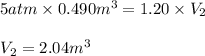Chemistry, 02.07.2019 03:10 ambriyaarmstrong01
Acontainer holds 0.490 m3 of oxygen at an absolute pressure of 5.00 atm. a valve is opened, allowing the gas to drive a piston, increasing the volume of the gas until the pressure drops to 1.20 atm. if the temperature remains constant, what new volume (in m3) does the gas occupy? hint m3

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, kristineford198
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3



Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
Acontainer holds 0.490 m3 of oxygen at an absolute pressure of 5.00 atm. a valve is opened, allowing...
Questions in other subjects:





History, 17.07.2020 22:01


Computers and Technology, 17.07.2020 22:01

Mathematics, 17.07.2020 22:01

Mathematics, 17.07.2020 22:01

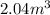
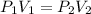 (at constant temperature)
(at constant temperature)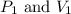 are initial pressure and volume.
are initial pressure and volume.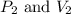 are final pressure and volume.
are final pressure and volume.