
Chemistry, 02.07.2019 03:10 wedderman6049
You have 3.00 l of a 3.00 m solution of nacl(aq) called solution a. you also have 2.00 l of a 2.00 m solution of agno3(aq) called solution b. you mix these solutions together, making solution c. hint: agcl is a precipitate. calculate the concentrations (in m) of the following ions in solution c. no3-

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 12:00, ctyrector
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 12:40, jaylen2559
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1
You know the right answer?
You have 3.00 l of a 3.00 m solution of nacl(aq) called solution a. you also have 2.00 l of a 2.00 m...
Questions in other subjects:


Geography, 22.02.2021 18:40





Mathematics, 22.02.2021 18:40

English, 22.02.2021 18:40


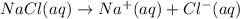
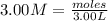
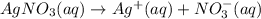
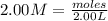
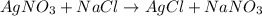
![[NO_{3}^-]=\frac{4 mol}{5 L}=0.8 mol/L](/tpl/images/0041/0466/43242.png)


