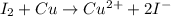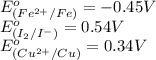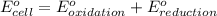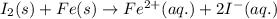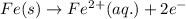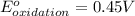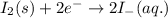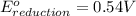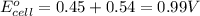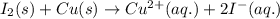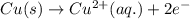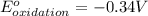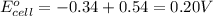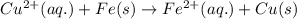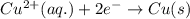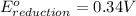Chemistry, 02.07.2019 00:20 cportillo891
Combine the two half-reactions that give the spontaneous cell reaction with the smallest e∘. fe2+(aq)+2e−→fe(s) e∘=−0.45v i2(s)+2e−→2i−(aq) e∘=0.54v cu2+(aq)+2e−→cu(s) e∘=0.34v

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, chrisxxxrv24
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 01:30, alfarodougoy8lvt
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3
You know the right answer?
Combine the two half-reactions that give the spontaneous cell reaction with the smallest e∘. fe2+(aq...
Questions in other subjects:

Mathematics, 29.11.2020 14:00

History, 29.11.2020 14:00

Computers and Technology, 29.11.2020 14:00

Arts, 29.11.2020 14:00

English, 29.11.2020 14:00

Mathematics, 29.11.2020 14:00


Mathematics, 29.11.2020 14:00


Mathematics, 29.11.2020 14:00

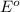 is
is