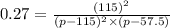Chemistry, 01.07.2019 23:30 NeverEndingCycle
Nitric oxide reacts with chlorine gas according to the reaction: 2 no( g) + cl2( g) ∆ 2 nocl( g) kp = 0.27 at 700 k a reaction mixture initially contains equal partial pressures of no and cl2. at equilibrium, the partial pressure of nocl is 115 torr. what were the initial partial pressures of no and cl2 ?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, jordan5778
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 15:00, kandi2565
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
You know the right answer?
Nitric oxide reacts with chlorine gas according to the reaction: 2 no( g) + cl2( g) ∆ 2 nocl( g) kp...
Questions in other subjects:

Law, 07.09.2021 14:00

English, 07.09.2021 14:00

Mathematics, 07.09.2021 14:00

Mathematics, 07.09.2021 14:00

English, 07.09.2021 14:00


Social Studies, 07.09.2021 14:00

Mathematics, 07.09.2021 14:00


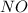 and
and  is 139.4 torr.
is 139.4 torr. 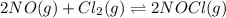
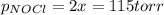
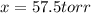
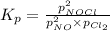
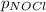 = 2x =115 torr
= 2x =115 torr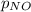 = (p-2x) = p-115 torr
= (p-2x) = p-115 torr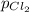 = (p-x)= p-57.5 torr
= (p-x)= p-57.5 torr