
Chemistry, 01.07.2019 22:40 ritahastie7533
The zero order reaction 2n2o→2n2+o2 has the reaction constant k is 6.28×10−3 moll s. if the initial concentration of n2o is 0.962 mol/l, what is the concentration of n2o after 10.0 seconds? your answer should have three significant figures (three decimal places).

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 18:00, ambarpena14
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 22:40, destineysarah
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
The zero order reaction 2n2o→2n2+o2 has the reaction constant k is 6.28×10−3 moll s. if the initial...
Questions in other subjects:

Mathematics, 23.02.2021 07:50




Mathematics, 23.02.2021 07:50


History, 23.02.2021 07:50


Mathematics, 23.02.2021 07:50

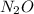 in three significant figures will be 0.899 mol/L.
in three significant figures will be 0.899 mol/L.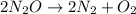
![k=\frac{1}{t}([A_o]-[A])](/tpl/images/0040/3296/5bd85.png)
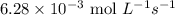
![[A_o]](/tpl/images/0040/3296/dc622.png) = initial concentration of the reactant = 0.962 mol/L
= initial concentration of the reactant = 0.962 mol/L![6.28\times 10^{-3}=\frac{1}{10}(0.962-[A])](/tpl/images/0040/3296/18cd6.png)
![[A]=0.899mol/L](/tpl/images/0040/3296/6a562.png)



