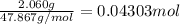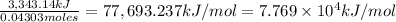Chemistry, 01.07.2019 21:30 faithkristi
The combustion of titanium with oxygen produces titanium dioxide:
ti (s) + o2 (g) → tio2 (s)
when 2.060 g of titanium is combusted in a bomb calorimeter, the temperature of the calorimeter increases from 25.00 °c to 91.60 °c. in a separate experiment, the heat capacity of the calorimeter is measured to be 9.84 kj/k. the heat of reaction for the combustion of a mole of ti in this calorimeter is kj/mol.
ti = 47.867 amu
o2 = 31.9988 amu
tio2 = 79.8650 amu
report answer in scientific notation use en rather than x 10n

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1


Chemistry, 22.06.2019 21:00, nsutton9985
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

You know the right answer?
The combustion of titanium with oxygen produces titanium dioxide:
ti (s) + o2 (g) → tio2 (s)<...
ti (s) + o2 (g) → tio2 (s)<...
Questions in other subjects:



Social Studies, 09.04.2022 21:30





History, 09.04.2022 21:40


Biology, 09.04.2022 21:50

 .
.