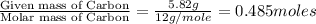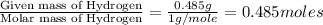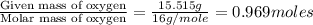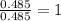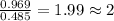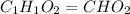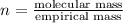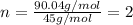Chemistry, 01.07.2019 21:20 robert7248
A21.82 gram sample of an organic compound containing c, h and o is analyzed by combustion analysis and 21.33 grams of co2 and 4.366 grams of h2o are produced. in a separate experiment, the molar mass is found to be 90.04 g/mol. determine the empirical formula and the molecular formula of the organic compound.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, dinosaur10
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 03:00, HHHHHHHHHMMMMMMMMM
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 03:30, jabper5522
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 05:00, YoEsMyles3115
0.2348 grams of pbcl2 used to form 44.0 ml of solution.
Answers: 1
You know the right answer?
A21.82 gram sample of an organic compound containing c, h and o is analyzed by combustion analysis a...
Questions in other subjects:




Mathematics, 16.04.2021 18:30

Mathematics, 16.04.2021 18:30

Arts, 16.04.2021 18:30



Biology, 16.04.2021 18:30

Mathematics, 16.04.2021 18:30

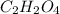
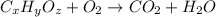
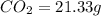
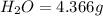
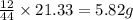 of carbon will be contained.
of carbon will be contained.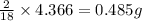 of hydrogen will be contained.
of hydrogen will be contained.