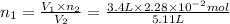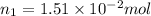Nitrogen dioxide is a red-brown gas responsible for the brown color of smog. a container of nitrogen dioxide that is at low pressure and at room temperature has a volume of 3.41l. after more nitrogen dioxide is added, the container holds 2.28×10−2mol of nitrogen dioxide and the volume of the container is 5.11l, still at the same pressure and temperature. how many moles of nitrogen dioxide were in the container initially? give your answer in three significant figures.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, johngayden46
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 21.06.2019 21:10, cordovamaria22
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1


Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
You know the right answer?
Nitrogen dioxide is a red-brown gas responsible for the brown color of smog. a container of nitrogen...
Questions in other subjects:

History, 24.02.2021 21:00




Mathematics, 24.02.2021 21:00

Mathematics, 24.02.2021 21:00




Mathematics, 24.02.2021 21:00

 of nitrogen dioxide were in the container .
of nitrogen dioxide were in the container .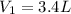

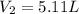

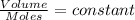 (at constant pressure and temperature)
(at constant pressure and temperature)