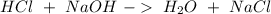The reactants of two chemical reactions are shown.
reaction 1: hcl and naoh
reaction 2:...

Chemistry, 01.07.2019 20:20 Nathaliasmiles
The reactants of two chemical reactions are shown.
reaction 1: hcl and naoh
reaction 2: cu and agno3
which reaction is likely to be a redox reaction?
a) reaction 1, because hydrogen and hydroxide atoms will be formed.
b) reaction 1, because all ions formed will retain their electrostatic charges.
c) reaction 2, because the electrostatic charge on copper ion will change.
d) reaction 2, because the charges on each side of the equation will be unequal.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1


Chemistry, 22.06.2019 21:30, thompsonhomes1
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

You know the right answer?
Questions in other subjects:


History, 09.09.2020 15:01

English, 09.09.2020 15:01

Mathematics, 09.09.2020 15:01


Mathematics, 09.09.2020 15:01