Chemistry, 01.07.2019 16:10 richardgibson2005
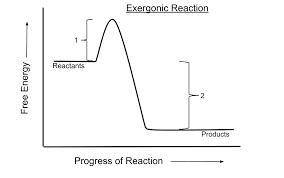
Which of the following is true for all exergonic reactions? the reaction releases energy. a net input of energy from the surroundings is required for the reactions to proceed. the reactions are rapid. the products have more total energy than the reactants. the reaction goes only in a forward direction: all reactants will be converted to products, but no products will be converted to reactants.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:20, datboyjulio21
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 13:00, nadiarose6345
In a copper wire, a temperature increase is the result of which of the following
Answers: 1
You know the right answer?
Which of the following is true for all exergonic reactions? the reaction releases energy. a net inp...
Questions in other subjects:

Social Studies, 22.07.2021 05:10



Mathematics, 22.07.2021 05:10


Chemistry, 22.07.2021 05:10

Social Studies, 22.07.2021 05:20

Social Studies, 22.07.2021 05:20