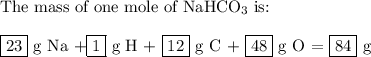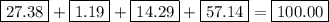Chemistry, 01.07.2019 00:10 lazerlemon500
(need all boxes answered)
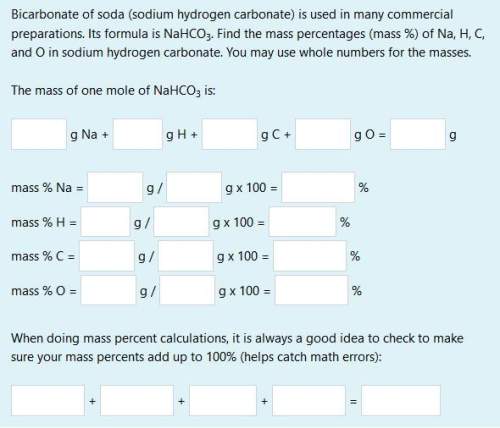
bicarbonate of soda (sodium hydrogen carbonate) is used in many commercial preparations. its formula is nahco3. find the mass percentages (mass %) of na, h, c, and o in sodium hydrogen carbonate. you may use whole numbers for the masses.
the mass of one mole of nahco3 is:

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, steven2996
What can be the use of smoke transformed into liquid?
Answers: 1


Chemistry, 22.06.2019 03:00, ian2006huang
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 03:00, cheesecake1919
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3
You know the right answer?
(need all boxes answered)
bicarbonate of soda (sodium hydrogen carbonate) is used in many comm...
bicarbonate of soda (sodium hydrogen carbonate) is used in many comm...
Questions in other subjects:

Mathematics, 21.06.2019 23:00

Geography, 21.06.2019 23:00



Physics, 21.06.2019 23:00


Physics, 21.06.2019 23:00

Mathematics, 21.06.2019 23:00